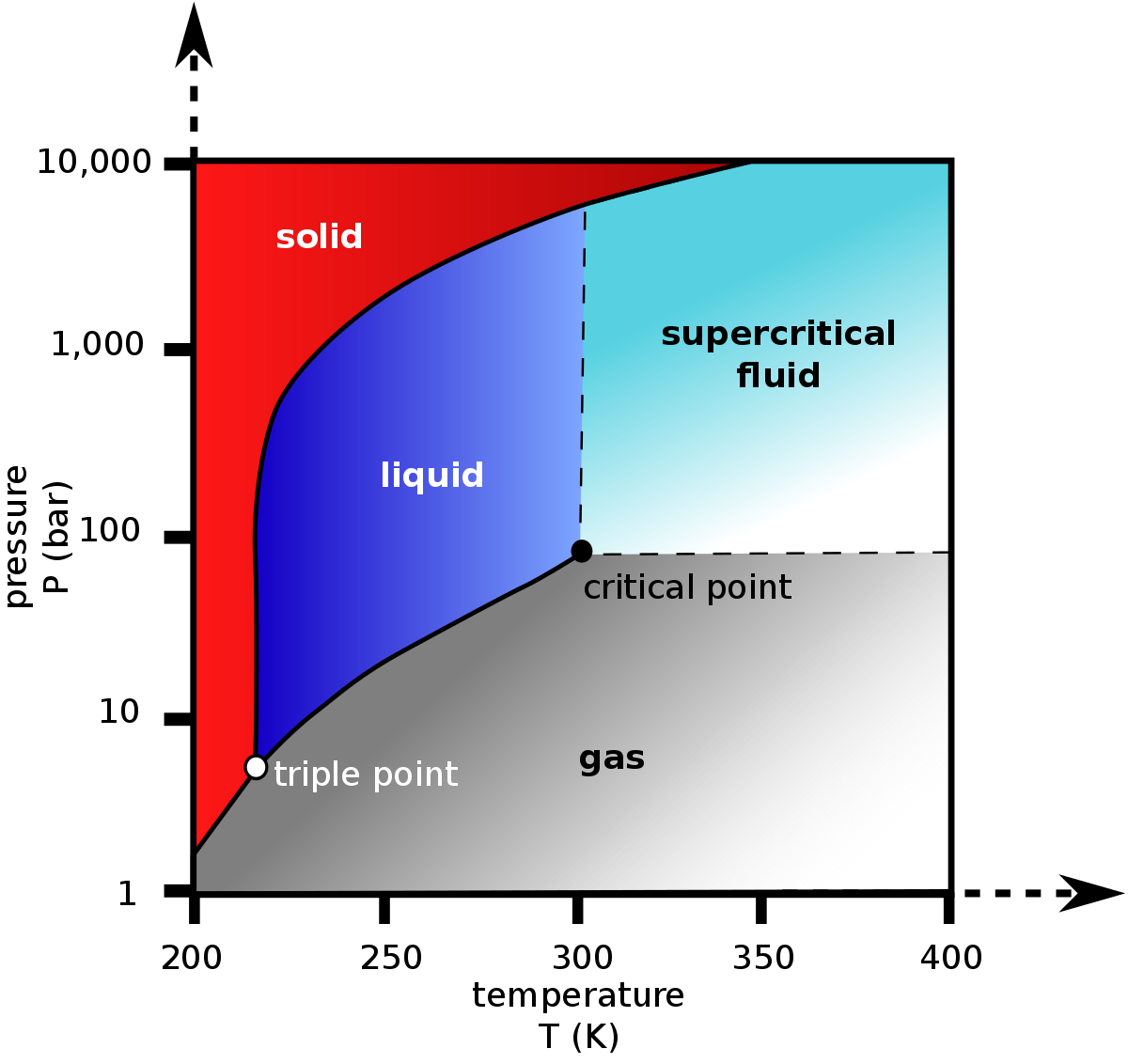
A type of chart used to show conditions (temperature, pressure, volume, etc.) at which phases (solids and fluids) occur and coexist at thermodynamic equilibrium.
One of the most popular chart for the Phase Equilibrium Diagram is PT Diagram (see Fig. 1 for pure substance and Fig. 2 for fluid mixtures).
| Fig. 1. Schematic PT diagram with Vapour Liquid Equilibrium curve for pure substance and Critical Point. | Fig. 2. Schematic PT diagram with Vapour Liquid Equilibrium region for fluid mixture and Pseudo-Critical Point. The dash lines showing the Vapour Quality Lines (with inverse numbering). |
See also
Natural Science / Physics / Thermodynamics / Thermodynamic system / Phase / Phase Equilibrium
[ State of matter ][ Pure substance ] [ Mixtures ][ Fluid Mixtures ]
[ Thermodynamic equilibrium ][ Vapour Liquid Equilibrium (VLE) ][ Gibbs Phase Rule ]